1. American Heart Association. (2021). What is High Blood Pressure? https://www.heart.org/-/media/Files/Health-Topics/Answers-by-Heart/What-Is-High-Blood-Pressure.pdf
2. Hörnfeldt, E., & Kahan, T. (2021, June). Hypertoni—Viss.nu [Text]. Viss.nu, Region Stockholm, Ett kunskapsstöd för dig som arbetar i primärvården. https://viss.nu/kunskapsstod/vardprogram/hypertoni
3. GBD Compare Data Visualization. (2020). Institute for Health Metrics and Evaluation (IHME), Seattle, WA: IHME, University of Washington, 2020. http://vizhub.healthdata.org/gbd-compare
4. Ismail, L., Materwala, H., & Al Kaabi, J. (2021). Association of risk factors with type 2 diabetes: A systematic review. Computational and Structural Biotechnology Journal, 19, 1759–1785. https://doi.org/10.1016/j.csbj.2021.03.003
5. Lewington, S., Clarke, R., Qizilbash, N., Peto, R., Collins, R., & Prospective Studies Collaboration. (2002). Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet (London, England), 360(9349), 1903–1913. https://doi.org/10.1016/s0140-6736(02)11911-8
6. Petrie, J. R., Guzik, T. J., & Touyz, R. M. (2018). Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. The Canadian Journal of Cardiology, 34(5), 575–584. https://doi.org/10.1016/j.cjca.2017.12.005
7. WHO fact sheet hypertension. (2021, August 21). World Health Organization, Fact Sheets, Hypertension. https://www.who.int/news-room/fact-sheets/detail/hypertension
8. Zhou, B., Carrillo-Larco, R. M., Danaei, G., Riley, L. M., Paciorek, C. J., Stevens, G. A., Gregg, E. W., Bennett, J. E., Solomon, B., Singleton, R. K., Sophiea, M. K., Iurilli, M. L., Lhoste, V. P., Cowan, M. J., Savin, S., Woodward, M., Balanova, Y., Cifkova, R., Damasceno, A., … Ezzati, M. (2021). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. The Lancet, 398(10304), 957–980. https://doi.org/10.1016/S0140-6736(21)01330-1
9. Balu, S. (2009). Estimated annual direct expenditures in the United States as a result of inappropriate hypertension treatment according to national treatment guidelines. Clinical Therapeutics, 31(7), 1581–1594. https://doi.org/10.1016/j.clinthera.2009.07.010
10. Balu, S., & Thomas, J. (2006). Incremental expenditure of treating hypertension in the United States. American Journal of Hypertension, 19(8), 810–816; discussion 817. https://doi.org/10.1016/j.amjhyper.2005.12.013
11. Benjamin, E. J., Blaha, M. J., Chiuve, S. E., Cushman, M., Das, S. R., Deo, R., de Ferranti, S. D., Floyd, J., Fornage, M., Gillespie, C., Isasi, C. R., Jiménez, M. C., Jordan, L. C., Judd, S. E., Lackland, D., Lichtman, J. H., Lisabeth, L., Liu, S., Longenecker, C. T., … American Heart Association Statistics Committee and Stroke Statistics Subcommittee. (2017). Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation, 135(10), e146–e603. https://doi.org/10.1161/CIR.0000000000000485
12. Davis, K. E. (2013). Expenditures for Hypertension among Adults Age 18 and Older, 2010: Estimates for the U.S. Civilian Noninstitutionalized Population. In Statistical Brief (Medical Expenditure Panel Survey (US)). Agency for Healthcare Research and Quality (US). http://www.ncbi.nlm.nih.gov/books/NBK525008/
13. Nakamura, K., Okamura, T., Miura, K., & Okayama, A. (2014). Hypertension and medical expenditure in the Japanese population: Review of prospective studies. World Journal of Cardiology, 6(7), 531–538. https://doi.org/10.4330/wjc.v6.i7.531
14. Dai, H., Much, A. A., Maor, E., Asher, E., Younis, A., Xu, Y., Lu, Y., Liu, X., Shu, J., & Bragazzi, N. L. (2022). Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990-2017: Results from the Global Burden of Disease Study 2017. European Heart Journal. Quality of Care & Clinical Outcomes, 8(1), 50–60. https://doi.org/10.1093/ehjqcco/qcaa076
15. Odden, M. C., Coxson, P. G., Moran, A., Lightwood, J. M., Goldman, L., & Bibbins-Domingo, K. (2011). The impact of the aging population on coronary heart disease in the United States. The American Journal of Medicine, 124(9), 827-833.e5. https://doi.org/10.1016/j.amjmed.2011.04.010
16. Cook, C., Cole, G., Asaria, P., Jabbour, R., & Francis, D. P. (2014). The annual global economic burden of heart failure. International Journal of Cardiology, 171(3), 368–376. https://doi.org/10.1016/j.ijcard.2013.12.028
17. Galis, Z. S., Thrasher, T., Reid, D. M., Stanley, D. V., & Oh, Y. S. (2013). Investing in high blood pressure research: A national institutes of health perspective. Hypertension (Dallas, Tex.: 1979), 61(4), 757–761. https://doi.org/10.1161/HYPERTENSIONAHA.111.00770
18. Dzau, V. J., & Balatbat, C. A. (2019). Future of Hypertension. Hypertension (Dallas, Tex.: 1979), 74(3), 450–457. https://doi.org/10.1161/HYPERTENSIONAHA.119.13437
19. Daugherty, A. M. (2021). Hypertension-related risk for dementia: A summary review with future directions. Seminars in Cell & Developmental Biology, 116, 82–89. https://doi.org/10.1016/j.semcdb.2021.03.002
20. Derington, C. G., King, J. B., Bryant, K. B., McGee, B. T., Moran, A. E., Weintraub, W. S., Bellows, B. K., & Bress, A. P. (2019). Cost-Effectiveness and Challenges of Implementing Intensive Blood Pressure Goals and Team-Based Care. Current Hypertension Reports, 21(12), 91. https://doi.org/10.1007/s11906-019-0996-x
21. Marchi, K. C., Muniz, J. J., & Tirapelli, C. R. (2014). Hypertension and chronic ethanol consumption: What do we know after a century of study? World Journal of Cardiology, 6(5), 283–294. https://doi.org/10.4330/wjc.v6.i5.283
22. Goslawski, M., Piano, M. R., Bian, J.-T., Church, E. C., Szczurek, M., & Phillips, S. A. (2013). Binge drinking impairs vascular function in young adults. Journal of the American College of Cardiology, 62(3), 201–207. https://doi.org/10.1016/j.jacc.2013.03.049
23. Oda, N., Kajikawa, M., Maruhashi, T., Iwamoto, Y., Kishimoto, S., Matsui, S., Hidaka, T., Kihara, Y., Chayama, K., Goto, C., Aibara, Y., Nakashima, A., Noma, K., Tomiyama, H., Takase, B., Yamashina, A., & Higashi, Y. (2017). Endothelial function is impaired in relation to alcohol intake even in the case of light alcohol consumption in Asian men; Flow-mediated Dilation Japan (FMD-J) Study. International Journal of Cardiology, 230, 523–528. https://doi.org/10.1016/j.ijcard.2016.12.065
24. Suzuki, K., Elkind, M. S. V., Boden-Albala, B., Jin, Z., Berry, G., Di Tullio, M. R., Sacco, R. L., & Homma, S. (2009). Moderate alcohol consumption is associated with better endothelial function: A cross sectional study. BMC Cardiovascular Disorders, 9, 8. https://doi.org/10.1186/1471-2261-9-8
25. Phillips, S. A., Osborn, K., Hwang, C.-L., Sabbahi, A., & Piano, M. R. (2020). Ethanol Induced Oxidative Stress in the Vasculature: Friend or Foe. Current Hypertension Reviews, 16(3), 181–191. https://doi.org/10.2174/1573402115666190325124622
26. Hwang, C.-L., Muchira, J., Hibner, B. A., Phillips, S. A., & Piano, M. R. (2022). Alcohol Consumption: A New Risk Factor for Arterial Stiffness? Cardiovascular Toxicology, 22(3), 236–245. https://doi.org/10.1007/s12012-022-09728-8
27. Laurent, S., Boutouyrie, P., Asmar, R., Gautier, I., Laloux, B., Guize, L., Ducimetiere, P., & Benetos, A. (2001). Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension (Dallas, Tex.: 1979), 37(5), 1236–1241. https://doi.org/10.1161/01.hyp.37.5.1236
28. Laurent, S., Katsahian, S., Fassot, C., Tropeano, A.-I., Gautier, I., Laloux, B., & Boutouyrie, P. (2003). Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke, 34(5), 1203–1206. https://doi.org/10.1161/01.STR.0000065428.03209.64
29. Del Giorno, R., Maddalena, A., Bassetti, S., & Gabutti, L. (2022). Association between Alcohol Intake and Arterial Stiffness in Healthy Adults: A Systematic Review. Nutrients, 14(6), 1207. https://doi.org/10.3390/nu14061207
30. Charakida, M., Georgiopoulos, G., Dangardt, F., Chiesa, S. T., Hughes, A. D., Rapala, A., Davey Smith, G., Lawlor, D., Finer, N., & Deanfield, J. E. (2019). Response to ‘Does smoking or alcohol cause early vascular damage in teenage years?’ European Heart Journal, 40(42), 3497. https://doi.org/10.1093/eurheartj/ehz608
31. El Khoudary, S. R., Barinas-Mitchell, E., White, J., Sutton-Tyrrell, K., Kuller, L. H., Curb, J. D., Shin, C., Ueshima, H., Masaki, K., Evans, R. W., Miura, K., Edmundowicz, D., Sekikawa, A., & ERA JUMP Study Group. (2012). Adiponectin, systolic blood pressure, and alcohol consumption are associated with more aortic stiffness progression among apparently healthy men. Atherosclerosis, 225(2), 475–480. https://doi.org/10.1016/j.atherosclerosis.2012.09.015
32. Gonzalez-Sanchez, J., Garcia-Ortiz, L., Rodriguez-Sanchez, E., Maderuelo-Fernandez, J. A., Tamayo-Morales, O., Lugones-Sanchez, C., Recio-Rodriguez, J. I., Gomez-Marcos, M. A., & EVA Investigators. (2020). The Relationship Between Alcohol Consumption With Vascular Structure and Arterial Stiffness in the Spanish Population: EVA Study. Alcoholism, Clinical and Experimental Research, 44(9), 1816–1824. https://doi.org/10.1111/acer.14411
33. Hwang, C.-L., Piano, M. R., Thur, L. A., Peters, T. A., da Silva, A. L. G., & Phillips, S. A. (2020). The effects of repeated binge drinking on arterial stiffness and urinary norepinephrine levels in young adults. Journal of Hypertension, 38(1), 111–117. https://doi.org/10.1097/HJH.0000000000002223
34. O’Neill, D., Britton, A., Brunner, E. J., & Bell, S. (2017). Twenty-Five-Year Alcohol Consumption Trajectories and Their Association With Arterial Aging: A Prospective Cohort Study. Journal of the American Heart Association, 6(2), e005288. https://doi.org/10.1161/JAHA.116.005288
35. Shiina, K., Takahashi, T., Nakano, H., Fujii, M., Iwasaki, Y., Matsumoto, C., Yamashina, A., Chikamori, T., & Tomiyama, H. (2022). Longitudinal Associations between Alcohol Intake and Arterial Stiffness, Pressure Wave Reflection, and Inflammation. Journal of Atherosclerosis and Thrombosis. https://doi.org/10.5551/jat.63544
36. Tisdel, D. M., Gadberry, J. J., Burke, S. L., Carlini, N. A., Fleenor, B. S., & Campbell, M. S. (2021). Dietary fat and alcohol in the prediction of indices of vascular health among young adults. Nutrition (Burbank, Los Angeles County, Calif.), 84, 111120. https://doi.org/10.1016/j.nut.2020.111120
37. Fitzpatrick, E., Han, X., Liu, W., Corcoran, E., Burtenshaw, D., Morrow, D., Helt, J.-C., Cahill, P. A., & Redmond, E. M. (2017). Alcohol Reduces Arterial Remodeling by Inhibiting Sonic Hedgehog-Stimulated Stem Cell Antigen-1 Positive Progenitor Stem Cell Expansion. Alcoholism, Clinical and Experimental Research, 41(12), 2051–2065. https://doi.org/10.1111/acer.13499
38. Liu, W., Harman, S., DiLuca, M., Burtenshaw, D., Corcoran, E., Cahill, P. A., & Redmond, E. M. (2020). Moderate Alcohol Consumption Targets S100ß+ Vascular Stem Cells and Attenuates Injury-Induced Neointimal Hyperplasia. Alcoholism, Clinical and Experimental Research, 44(9), 1734–1746. https://doi.org/10.1111/acer.14415
39. J uonala, M., Viikari, J. S. A., Kähönen, M., Laitinen, T., Taittonen, L., Loo, B.-M., Jula, A., Marniemi, J., Räsänen, L., Rönnemaa, T., & Raitakari, O. T. (2009). Alcohol consumption is directly associated with carotid intima-media thickness in Finnish young adults: The Cardiovascular Risk in Young Finns Study. Atherosclerosis, 204(2), e93-98. https://doi.org/10.1016/j.atherosclerosis.2008.11.021
40. Britton, A. R., Grobbee, D. E., den Ruijter, H. M., Anderson, T. J., Desvarieux, M., Engström, G., Evans, G. W., Hedblad, B., Kauhanen, J., Kurl, S., Lonn, E. M., Mathiesen, E. B., Polak, J. F., Price, J. F., Rembold, C. M., Rosvall, M., Rundek, T., Salonen, J. T., Stehouwer, C., … Bots, M. L. (2017). Alcohol Consumption and Common Carotid Intima-Media Thickness: The USE-IMT Study. Alcohol and Alcoholism (Oxford, Oxfordshire), 52(4), 483–486. https://doi.org/10.1093/alcalc/agx028
41. Mahajan, H., Choo, J., Masaki, K., Fujiyoshi, A., Guo, J., Hisamatsu, T., Evans, R., Shangguan, S., Willcox, B., Okamura, T., Vishnu, A., Barinas-Mitchell, E., Ahuja, V., Miura, K., Kuller, L., Shin, C., Ueshima, H., & Sekikawa, A. (2018). Association of alcohol consumption and aortic calcification in healthy men aged 40-49 years for the ERA JUMP Study. Atherosclerosis, 268, 84–91. https://doi.org/10.1016/j.atherosclerosis.2017.11.017
42. Pletcher, M. J., Varosy, P., Kiefe, C. I., Lewis, C. E., Sidney, S., & Hulley, S. B. (2005). Alcohol consumption, binge drinking, and early coronary calcification: Findings from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. American Journal of Epidemiology, 161(5), 423–433. https://doi.org/10.1093/aje/kwi062
43. Yun, K. E., Chang, Y., Yun, S.-C., Davey Smith, G., Ryu, S., Cho, S.-I., Chung, E. C., Shin, H., & Khang, Y.-H. (2017). Alcohol and coronary artery calcification: An investigation using alcohol flushing as an instrumental variable. International Journal of Epidemiology, 46(3), 950–962. https://doi.org/10.1093/ije/dyw237
44. Yang, Y., Zhang, N., Huang, W., Feng, R., Feng, P., Gu, J., Liu, G., & Lei, H. (2017). The relationship of alcohol consumption with left ventricular mass in people 35 years old or older in rural areas of Western China. Journal of the American Society of Hypertension: JASH, 11(4), 220–226. https://doi.org/10.1016/j.jash.2017.02.002
45. Catena, C., Colussi, G., Verheyen, N. D., Novello, M., Fagotto, V., Soardo, G., & Sechi, L. A. (2016). Moderate Alcohol Consumption Is Associated With Left Ventricular Diastolic Dysfunction in Nonalcoholic Hypertensive Patients. Hypertension (Dallas, Tex.: 1979), 68(5), 1208–1216. https://doi.org/10.1161/HYPERTENSIONAHA.116.08145
46. Julian, T. H., Syeed, R., Glascow, N., & Zis, P. (2020). Alcohol-induced autonomic dysfunction: A systematic review. Clinical Autonomic Research, 30(1), 29–41. https://doi.org/10.1007/s10286-019-00618-8
47. Tasnim, S., Tang, C., Musini, V. M., & Wright, J. M. (2020). Effect of alcohol on blood pressure. The Cochrane Database of Systematic Reviews, 7, CD012787. https://doi.org/10.1002/14651858.CD012787.pub2
48. Greenlund, I. M., Cunningham, H. A., Tikkanen, A. L., Bigalke, J. A., Smoot, C. A., Durocher, J. J., & Carter, J. R. (2021). Morning sympathetic activity after evening binge alcohol consumption. American Journal of Physiology. Heart and Circulatory Physiology, 320(1), H305–H315. https://doi.org/10.1152/ajpheart.00743.2020
49. Roerecke, M., Kaczorowski, J., Tobe, S. W., Gmel, G., Hasan, O. S. M., & Rehm, J. (2017). The effect of a reduction in alcohol consumption on blood pressure: A systematic review and meta-analysis. The Lancet. Public Health, 2(2), e108–e120. https://doi.org/10.1016/S2468-2667(17)30003-8
50. Blalock, D. V., Berlin, S. A., Young, J. R., Blakey, S. M., Calhoun, P. S., & Dedert, E. A. (2022). Effects of Alcohol Reduction Interventions on Blood Pressure. Current Hypertension Reports, 24(4), 75–85. https://doi.org/10.1007/s11906-022-01171-y
51. Kabayama, M., Akagi, Y., Wada, N., Higuchi, A., Tamatani, M., Tomita, J., Nakata, Y., Takiuchi, S., Yamamoto, K., Sugimoto, K., Shintani, A., Rakugi, H., & Kamide, K. (2021). A Randomized Trial of Home Blood-Pressure Reduction by Alcohol Guidance During Outpatient Visits: OSAKE Study. American Journal of Hypertension, 34(10), 1108–1115. https://doi.org/10.1093/ajh/hpab082
52. Stewart, S. H., Latham, P. K., Miller, P. M., Randall, P., & Anton, R. F. (2008). Blood pressure reduction during treatment for alcohol dependence: Results from the Combining Medications and Behavioral Interventions for Alcoholism (COMBINE) study. Addiction (Abingdon, England), 103(10), 1622–1628. https://doi.org/10.1111/j.1360-0443.2008.02317.x
53. Witkiewitz, K., Kranzler, H. R., Hallgren, K. A., O’Malley, S. S., Falk, D. E., Litten, R. Z., Hasin, D. S., Mann, K. F., & Anton, R. F. (2018). Drinking Risk Level Reductions Associated with Improvements in Physical Health and Quality of Life Among Individuals with Alcohol Use Disorder. Alcoholism, Clinical and Experimental Research, 42(12), 2453–2465. https://doi.org/10.1111/acer.13897
54. Xin, X., He, J., Frontini, M. G., Ogden, L. G., Motsamai, O. I., & Whelton, P. K. (2001). Effects of alcohol reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension (Dallas, Tex.: 1979), 38(5), 1112–1117. https://doi.org/10.1161/hy1101.093424
55. Gepner, Y., Golan, R., Harman-Boehm, I., Henkin, Y., Schwarzfuchs, D., Shelef, I., Durst, R., Kovsan, J., Bolotin, A., Leitersdorf, E., Shpitzen, S., Balag, S., Shemesh, E., Witkow, S., Tangi-Rosental, O., Chassidim, Y., Liberty, I. F., Sarusi, B., Ben-Avraham, S., … Shai, I. (2015). Effects of Initiating Moderate Alcohol Intake on Cardiometabolic Risk in Adults With Type 2 Diabetes: A 2-Year Randomized, Controlled Trial. Annals of Internal Medicine, 163(8), 569–579. https://doi.org/10.7326/M14-1650
56. Chen, L., Smith, G. D., Harbord, R. M., & Lewis, S. J. (2008). Alcohol intake and blood pressure: A systematic review implementing a Mendelian randomization approach. PLoS Medicine, 5(3), e52. https://doi.org/10.1371/journal.pmed.0050052
57. Cho, Y., Shin, S.-Y., Won, S., Relton, C. L., Davey Smith, G., & Shin, M.-J. (2015). Alcohol intake and cardiovascular risk factors: A Mendelian randomisation study. Scientific Reports, 5, 18422. https://doi.org/10.1038/srep18422
58. Chang, Y.-C., Chiu, Y.-F., Lee, I.-T., Ho, L.-T., Hung, Y.-J., Hsiung, C. A., Quertermous, T., Donlon, T., Lee, W.-J., Lee, P.-C., Chen, C.-H., Mochly-Rosen, D., & Chuang, L.-M. (2012). Common ALDH2 genetic variants predict development of hypertension in the SAPPHIRe prospective cohort: Gene-environmental interaction with alcohol consumption. BMC Cardiovascular Disorders, 12, 58. https://doi.org/10.1186/1471-2261-12-58
59. Au Yeung, S. L., Jiang, C., Cheng, K. K., Cowling, B. J., Liu, B., Zhang, W., Lam, T. H., Leung, G. M., & Schooling, C. M. (2013). Moderate alcohol use and cardiovascular disease from Mendelian randomization. PloS One, 8(7), e68054. https://doi.org/10.1371/journal.pone.0068054
60. Millwood, I. Y., Walters, R. G., Mei, X. W., Guo, Y., Yang, L., Bian, Z., Bennett, D. A., Chen, Y., Dong, C., Hu, R., Zhou, G., Yu, B., Jia, W., Parish, S., Clarke, R., Davey Smith, G., Collins, R., Holmes, M. V., Li, L., … China Kadoorie Biobank Collaborative Group. (2019). Conventional and genetic evidence on alcohol and vascular disease aetiology: A prospective study of 500 000 men and women in China. Lancet (London, England), 393(10183), 1831–1842. https://doi.org/10.1016/S0140-6736(18)31772-0
61. Lawlor, D. A., Nordestgaard, B. G., Benn, M., Zuccolo, L., Tybjaerg-Hansen, A., & Davey Smith, G. (2013). Exploring causal associations between alcohol and coronary heart disease risk factors: Findings from a Mendelian randomization study in the Copenhagen General Population Study. European Heart Journal, 34(32), 2519–2528. https://doi.org/10.1093/eurheartj/eht081
62. Holmes, M. V., Dale, C. E., Zuccolo, L., Silverwood, R. J., Guo, Y., Ye, Z., Prieto-Merino, D., Dehghan, A., Trompet, S., Wong, A., Cavadino, A., Drogan, D., Padmanabhan, S., Li, S., Yesupriya, A., Leusink, M., Sundstrom, J., Hubacek, J. A., Pikhart, H., … on behalf of The InterAct Consortium. (2014). Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ, 349(jul10 6), g4164–g4164. https://doi.org/10.1136/bmj.g4164
63. Lankester, J., Zanetti, D., Ingelsson, E., & Assimes, T. L. (2021). Alcohol use and cardiometabolic risk in the UK Biobank: A Mendelian randomization study. PloS One, 16(8), e0255801. https://doi.org/10.1371/journal.pone.0255801
64. Roerecke, M., Tobe, S. W., Kaczorowski, J., Bacon, S. L., Vafaei, A., Hasan, O. S. M., Krishnan, R. J., Raifu, A. O., & Rehm, J. (2018). Sex-Specific Associations Between Alcohol Consumption and Incidence of Hypertension: A Systematic Review and Meta-Analysis of Cohort Studies. Journal of the American Heart Association, 7(13), e008202. https://doi.org/10.1161/JAHA.117.008202
65. Briasoulis, A., Agarwal, V., & Messerli, F. H. (2012). Alcohol consumption and the risk of hypertension in men and women: A systematic review and meta-analysis. Journal of Clinical Hypertension (Greenwich, Conn.), 14(11), 792–798. https://doi.org/10.1111/jch.12008
66. Taylor, B., Irving, H. M., Baliunas, D., Roerecke, M., Patra, J., Mohapatra, S., & Rehm, J. (2009). Alcohol and hypertension: Gender differences in dose-response relationships determined through systematic review and meta-analysis. Addiction (Abingdon, England), 104(12), 1981–1990. https://doi.org/10.1111/j.1360-0443.2009.02694.x
67. Puddey, I. B., Mori, T. A., Barden, A. E., & Beilin, L. J. (2019). Alcohol and Hypertension-New Insights and Lingering Controversies. Current Hypertension Reports, 21(10), 79. https://doi.org/10.1007/s11906-019-0984-1
68. Henriksson, K. M., Lindblad, U., Gullberg, B., Agren, B., Nilsson-Ehle, P., & Råstam, L. (2002). Development of hypertension over 6 years in a birth cohort of young middle-aged men: The Cardiovascular Risk Factor Study in southern Sweden (CRISS). Journal of Internal Medicine, 252(1), 21–26. https://doi.org/10.1046/j.1365-2796.2002.00996.x
69. Piano, M. R., Burke, L., Kang, M., & Phillips, S. A. (2018). Effects of Repeated Binge Drinking on Blood Pressure Levels and Other Cardiovascular Health Metrics in Young Adults: National Health and Nutrition Examination Survey, 2011-2014. Journal of the American Heart Association, 7(13), e008733. https://doi.org/10.1161/JAHA.118.008733
70. Hayibor, L. A., Zhang, J., & Duncan, A. (2019). Association of binge drinking in adolescence and early adulthood with high blood pressure: Findings from the National Longitudinal Study of Adolescent to Adult Health (1994-2008). Journal of Epidemiology and Community Health, 73(7), 652–659. https://doi.org/10.1136/jech-2018-211594
71. Wellman, R. J., Vaughn, J. A., Sylvestre, M.-P., O’Loughlin, E. K., Dugas, E. N., & O’Loughlin, J. L. (2016). Relationships Between Current and Past Binge Drinking and Systolic Blood Pressure in Young Adults. The Journal of Adolescent Health: Official Publication of the Society for Adolescent Medicine, 58(3), 352–357. https://doi.org/10.1016/j.jadohealth.2015.10.251
72. Khan, M. A., Hashim, M. J., Mustafa, H., Baniyas, M. Y., Al Suwaidi, S. K. B. M., AlKatheeri, R., Alblooshi, F. M. K., Almatrooshi, M. E. A. H., Alzaabi, M. E. H., Al Darmaki, R. S., & Lootah, S. N. A. H. (2020). Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus, 12(7), e9349. https://doi.org/10.7759/cureus.9349
73. Qureshi, N. Q., Mufarrih, S. H., Bloomfield, G. S., Tariq, W., Almas, A., Mokdad, A. H., Bartlett, J., Nisar, I., Siddiqi, S., Bhutta, Z., Mark, D., Douglas, P. S., & Samad, Z. (2021). Disparities in Cardiovascular Research Output and Disease Outcomes among High-, Middle- and Low-Income Countries – An Analysis of Global Cardiovascular Publications over the Last Decade (2008–2017). Global Heart, 16(1), 4. https://doi.org/10.5334/gh.815
74. Socialstyrelsen. (2022). Statistics on Causes of Death 2021 (p. 6). Socialstyrelsen. https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2022-6-8021.pdf
75. GBD 2019 Risk Factors Collaborators. (2020). Global burden of 87 risk factors in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England), 396(10258), 1223–1249. https://doi.org/10.1016/S0140-6736(20)30752-2
76. Ischemic Heart Disease—An overview | ScienceDirect Topics. (2022). Ischemic Heart Disease – an Overview. https://www.sciencedirect.com/topics/medicine-and-dentistry/ischemic-heart-disease
77. Andréasson, S., Chikritzhs, T., Dangardt, F., Holder, H., Naimi, T., & Stockwell, T. (2014). The Effects of Low-dose Alcohol Consumption (Alcohol and Society, pp. 6–23). http://urn.kb.se/resolve?urn=urn:nbn:se:iogt-2014-aos-en
78. Arora, M., ElSayed, A., Beger, B., Naidoo, P., Shilton, T., Jain, N., Armstrong-Walenczak, K., Mwangi, J., Wang, Y., Eiselé, J.-L., Pinto, F. J., & Champagne, B. M. (2022). The Impact of Alcohol Consumption on Cardiovascular Health: Myths and Measures. Global Heart, 17(1), 45. https://doi.org/10.5334/gh.1132
79. Griswold, M. G., Fullman, N., Hawley, C., Arian, N., Zimsen, S. R. M., Tymeson, H. D., Venkateswaran, V., Tapp, A. D., Forouzanfar, M. H., Salama, J. S., Abate, K. H., Abate, D., Abay, S. M., Abbafati, C., Abdulkader, R. S., Abebe, Z., Aboyans, V., Abrar, M. M., Acharya, P., … Gakidou, E. (2018). Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet, 392(10152), 1015–1035. https://doi.org/10.1016/S0140-6736(18)31310-2
80. Roerecke, M., & Rehm, J. (2012). The cardioprotective association of average alcohol consumption and ischaemic heart disease: A systematic review and meta-analysis: Alcohol and ischaemic heart disease-a meta-analysis. Addiction, 107(7), 1246–1260. https://doi.org/10.1111/j.1360-0443.2012.03780.x
81. Roerecke, M., & Rehm, J. (2013). What is Best Evidence in Epidemiology? A Reply to Stockwell (2012): The journal publishes both invited and unsolicited letters. Addiction, 108(2), 427–428. https://doi.org/10.1111/j.1360-0443.2012.04051.x
82. Zhao, J., Stockwell, T., Roemer, A., Naimi, T., & Chikritzhs, T. (2017). Alcohol Consumption and Mortality From Coronary Heart Disease: An Updated Meta-Analysis of Cohort Studies. Journal of Studies on Alcohol and Drugs, 78(3), 375–386. https://doi.org/10.15288/jsad.2017.78.375
83. Wallach, J. D., Serghiou, S., Chu, L., Egilman, A. C., Vasiliou, V., Ross, J. S., & Ioannidis, J. P. A. (2020). Evaluation of confounding in epidemiologic studies assessing alcohol consumption on the risk of ischemic heart disease. BMC Medical Research Methodology, 20(1), 64. https://doi.org/10.1186/s12874-020-0914-6
84. Brien, S. E., Ronksley, P. E., Turner, B. J., Mukamal, K. J., & Ghali, W. A. (2011). Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ, 342(feb22 1), d636–d636. https://doi.org/10.1136/bmj.d636
85. Chen, D., Zhang, Y., Yidilisi, A., Xu, Y., Dong, Q., & Jiang, J. (2022). Causal Associations Between Circulating Adipokines and Cardiovascular Disease: A Mendelian Randomization Study. The Journal of Clinical Endocrinology and Metabolism, 107(6), e2572–e2580. https://doi.org/10.1210/clinem/dgac048
86. Karjalainen, M. K., Holmes, M. V., Wang, Q., Anufrieva, O., Kähönen, M., Lehtimäki, T., Havulinna, A. S., Kristiansson, K., Salomaa, V., Perola, M., Viikari, J. S., Raitakari, O. T., Järvelin, M.-R., Ala-Korpela, M., & Kettunen, J. (2020). Apolipoprotein A-I concentrations and risk of coronary artery disease: A Mendelian randomization study. Atherosclerosis, 299, 56–63. https://doi.org/10.1016/j.atherosclerosis.2020.02.002
87. Richardson, T. G., Sanderson, E., Palmer, T. M., Ala-Korpela, M., Ference, B. A., Davey Smith, G., & Holmes, M. V. (2020). Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Medicine, 17(3), e1003062. https://doi.org/10.1371/journal.pmed.1003062
88. Sabater-Lleal, M., Huang, J., Chasman, D., Naitza, S., Dehghan, A., Johnson, A. D., Teumer, A., Reiner, A. P., Folkersen, L., Basu, S., Rudnicka, A. R., Trompet, S., Mälarstig, A., Baumert, J., Bis, J. C., Guo, X., Hottenga, J. J., Shin, S.-Y., Lopez, L. M., … O’Donnell, C. J. (2013). Multiethnic Meta-Analysis of Genome-Wide Association Studies in >100 000 Subjects Identifies 23 Fibrinogen-Associated Loci but No Strong Evidence of a Causal Association Between Circulating Fibrinogen and Cardiovascular Disease. Circulation, 128(12), 1310–1324. https://doi.org/10.1161/CIRCULATIONAHA.113.002251
89. Voight, B. F., Peloso, G. M., Orho-Melander, M., Frikke-Schmidt, R., Barbalic, M., Jensen, M. K., Hindy, G., Hólm, H., Ding, E. L., Johnson, T., Schunkert, H., Samani, N. J., Clarke, R., Hopewell, J. C., Thompson, J. F., Li, M., Thorleifsson, G., Newton-Cheh, C., Musunuru, K., … Kathiresan, S. (2012). Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. The Lancet, 380(9841), 572–580. https://doi.org/10.1016/S0140-6736(12)60312-2
90. Ward-Caviness, C. K., de Vries, P. S., Wiggins, K. L., Huffman, J. E., Yanek, L. R., Bielak, L. F., Giulianini, F., Guo, X., Kleber, M. E., Kacprowski, T., Groß, S., Petersman, A., Davey Smith, G., Hartwig, F. P., Bowden, J., Hemani, G., Müller-Nuraysid, M., Strauch, K., Koenig, W., … Morrison, A. C. (2019). Mendelian randomization evaluation of causal effects of fibrinogen on incident coronary heart disease. PloS One, 14(5), e0216222. https://doi.org/10.1371/journal.pone.0216222
91. Naimi, T. S., Stadtmueller, L. A., Chikritzhs, T., Stockwell, T., Zhao, J., Britton, A., Saitz, R., & Sherk, A. (2019). Alcohol, Age, and Mortality: Estimating Selection Bias Due to Premature Death. Journal of Studies on Alcohol and Drugs, 80(1), 63–68.
92. Fillmore, K. M., Stockwell, T., Chikritzhs, T., Bostrom, A., & Kerr, W. (2007). Moderate alcohol use and reduced mortality risk: Systematic error in prospective studies and new hypotheses. Annals of Epidemiology, 17(5 Suppl), S16-23. https://doi.org/10.1016/j.annepidem.2007.01.005
93. Liang, W., & Chikritzhs, T. (2013). The Association between Alcohol Exposure and Self-Reported Health Status: The Effect of Separating Former and Current Drinkers. PLoS ONE, 8(2), e55881. https://doi.org/10.1371/journal.pone.0055881
94. Lawes, C. M. M., Vander Hoorn, S., Rodgers, A., & International Society of Hypertension. (2008). Global burden of blood-pressure-related disease, 2001. Lancet (London, England), 371(9623), 1513–1518. https://doi.org/10.1016/S0140-6736(08)60655-8
95. Corrao, G., Bagnardi, V., Zambon, A., & La Vecchia, C. (2004). A meta-analysis of alcohol consumption and the risk of 15 diseases. Preventive Medicine, 38(5), 613–619. https://doi.org/10.1016/j.ypmed.2003.11.027
96. Piano, M. R. (2017). Alcohol’s Effects on the Cardiovascular System. Alcohol Research: Current Reviews, 38(2), 219–241.
97. Chikritzhs, T., Stockwell, T., Naimi, T., Andréasson, S., Dangardt, F., & Liang, W. (2015). Has the leaning tower of presumed health benefits from ‘moderate’ alcohol use finally collapsed?: Editorial. Addiction, 110(5), 726–727. https://doi.org/10.1111/add.12828
98. Ortolá, R., García-Esquinas, E., López-García, E., León-Muñoz, L. M., Banegas, J. R., & Rodríguez-Artalejo, F. (2019). Alcohol consumption and all-cause mortality in older adults in Spain: An analysis accounting for the main methodological issues. Addiction (Abingdon, England), 114(1), 59–68. https://doi.org/10.1111/add.14402
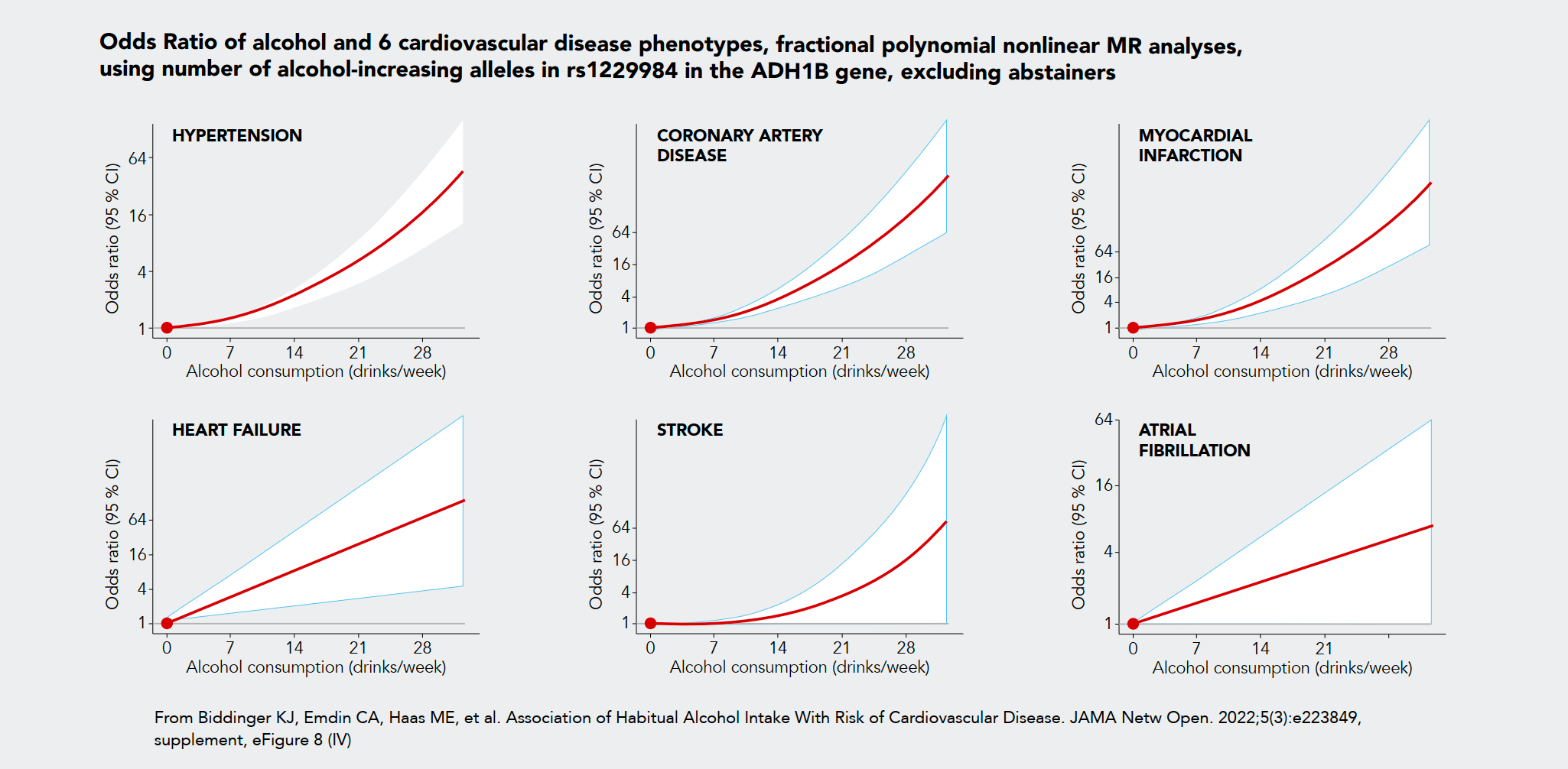
99. Biddinger, K. J., Emdin, C. A., Haas, M. E., Wang, M., Hindy, G., Ellinor, P. T., Kathiresan, S., Khera, A. V., & Aragam, K. G. (2022). Association of Habitual Alcohol Intake With Risk of Cardiovascular Disease. JAMA Network Open, 5(3), e223849. https://doi.org/10.1001/jamanetworkopen.2022.3849
100. Laonigro, I., Correale, M., Di Biase, M., & Altomare, E. (2009). Alcohol abuse and heart failure. European Journal of Heart Failure, 11(5), 453–462. https://doi.org/10.1093/eurjhf/hfp037
101. Manzo-Avalos, S., & Saavedra-Molina, A. (2010). Cellular and mitochondrial effects of alcohol consumption. International Journal of Environmental Research and Public Health, 7(12), 4281–4304. https://doi.org/10.3390/ijerph7124281
102. George, A., & Figueredo, V. M. (2011). Alcoholic cardiomyopathy: A review. Journal of Cardiac Failure, 17(10), 844–849. https://doi.org/10.1016/j.cardfail.2011.05.008
103. Piano, M. R. (2002). Alcoholic Cardiomyopathy. Chest, 121(5), 1638–1650. https://doi.org/10.1378/chest.121.5.1638
104. Rehm, J., Hasan, O. S. M., Imtiaz, S., & Neufeld, M. (2017). Quantifying the contribution of alcohol to cardiomyopathy: A systematic review. Alcohol (Fayetteville, N.Y.), 61, 9–15. https://doi.org/10.1016/j.alcohol.2017.01.011
105. Leon, D. A., Saburova, L., Tomkins, S., Andreev, E., Kiryanov, N., McKee, M., & Shkolnikov, V. M. (2007). Hazardous alcohol drinking and premature mortality in Russia: A population based case-control study. The Lancet, 369(9578), 2001–2009. https://doi.org/10.1016/S0140-6736(07)60941-6
106. Chugh, S. S., Havmoeller, R., Narayanan, K., Singh, D., Rienstra, M., Benjamin, E. J., Gillum, R. F., Kim, Y.-H., McAnulty, J. H., Zheng, Z.-J., Forouzanfar, M. H., Naghavi, M., Mensah, G. A., Ezzati, M., & Murray, C. J. L. (2014). Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation, 129(8), 837–847. https://doi.org/10.1161/CIRCULATIONAHA.113.005119
107. Kodama, S., Saito, K., Tanaka, S., Horikawa, C., Saito, A., Heianza, Y., Anasako, Y., Nishigaki, Y., Yachi, Y., Iida, K. T., Ohashi, Y., Yamada, N., & Sone, H. (2011). Alcohol consumption and risk of atrial fibrillation: A meta-analysis. Journal of the American College of Cardiology, 57(4), 427–436. https://doi.org/10.1016/j.jacc.2010.08.641
108. Larsson, S. C., Drca, N., & Wolk, A. (2014). Alcohol consumption and risk of atrial fibrillation: A prospective study and dose-response meta-analysis. Journal of the American College of Cardiology, 64(3), 281–289. https://doi.org/10.1016/j.jacc.2014.03.048
109. Voskoboinik, A., Prabhu, S., Ling, L.-H., Kalman, J. M., & Kistler, P. M. (2016). Alcohol and Atrial Fibrillation: A Sobering Review. Journal of the American College of Cardiology, 68(23), 2567–2576. https://doi.org/10.1016/j.jacc.2016.08.074
110. Rehm, J., Anderson, P., Prieto, J. A. A., Armstrong, I., Aubin, H.-J., Bachmann, M., Bastus, N. B., Brotons, C., Burton, R., Cardoso, M., Colom, J., Duprez, D., Gmel, G., Gual, A., Kraus, L., Kreutz, R., Liira, H., Manthey, J., Møller, L., … Zarco, J. (2017). Towards new recommendations to reduce the burden of alcohol-induced hypertension in the European Union. BMC Medicine, 15(1), 173. https://doi.org/10.1186/s12916-017-0934-1
111. Rehm, J., Gmel, G., Sierra, C., & Gual, A. (2018). Reduction of mortality following better detection of hypertension and alcohol problems in primary health care in Spain. Adicciones, 30(1), 9–18. https://doi.org/10.20882/adicciones.726
112. Hanschmidt, F., Manthey, J., Kraus, L., Scafato, E., Gual, A., Grimm, C., & Rehm, J. (2017). Barriers to Alcohol Screening Among Hypertensive Patients and the Role of Stigma: Lessons for the Implementation of Screening and Brief Interventions in European Primary Care Settings. Alcohol and Alcoholism (Oxford, Oxfordshire), 52(5), 572–579. https://doi.org/10.1093/alcalc/agx032
113. Bolbrinker, J., Zaidi Touis, L., Gohlke, H., Weisser, B., & Kreutz, R. (2018). European guidelines on lifestyle changes for management of hypertension: Awareness and implementation of recommendations among German and European physicians. Herz, 43(4), 352–358. https://doi.org/10.1007/s00059-017-4575-0
114. Miquel, L., López-Pelayo, H., Nuño, L., Arbesú, J. Á., Zarco, J., Manthey, J., Rehm, J., & Gual, A. (2018). Barriers to implement screening for alcohol consumption in Spanish hypertensive patients. Family Practice, 35(3), 295–301. https://doi.org/10.1093/fampra/cmx107
115. Zaidi Touis, L., Bolbrinker, J., Riemer, T. G., & Kreutz, R. (2018). Moderation of alcohol consumption as a recommendation in European hypertension management guidelines: A survey on awareness, screening and implementation among European physicians. BMJ Open, 8(10), e022026. https://doi.org/10.1136/bmjopen-2018-022026
116. Vay-Demouy, J., Lelong, H., Neudorff, P., Gabet, A., Grave, C., Blacher, J., & Olié, V. (2022). Underuse of lifestyle recommendations in hypertension management in France: The Esteban study. The Journal of Clinical Hypertension, jch.14576. https://doi.org/10.1111/jch.14576
117. Giesbrecht, N., Reisdorfer, E., & Rios, I. (2022). Alcohol Health Warning Labels: A Rapid Review with Action Recommendations. International Journal of Environmental Research and Public Health, 19(18), 11676. https://doi.org/10.3390/ijerph191811676
118. Health warning labels on alcoholic beverages: Opportunities for informed and healthier choices (Brief No. 4; Snapshot Series on Alcohol Control Policies and Practice). (2021). World Health Organization. https://www.who.int/publications-detail-redirect/9789240044449
119. Kokole, D., Anderson, P., & Jané-Llopis, E. (2021). Nature and Potential Impact of Alcohol Health Warning Labels: A Scoping Review. Nutrients, 13(9), 3065. https://doi.org/10.3390/nu13093065
120. Hobin, E., Weerasinghe, A., Vallance, K., Hammond, D., McGavock, J., Greenfield, T. K., Schoueri-Mychasiw, N., Paradis, C., & Stockwell, T. (2020). Testing Alcohol Labels as a Tool to Communicate Cancer Risk to Drinkers: A Real-World Quasi-Experimental Study. Journal of Studies on Alcohol and Drugs, 81(2), 249–261. https://doi.org/10.15288/jsad.2020.81.249
121. Schoueri-Mychasiw, N., Weerasinghe, A., Vallance, K., Stockwell, T., Zhao, J., Hammond, D., McGavock, J., Greenfield, T. K., Paradis, C., & Hobin, E. (2020). Examining the Impact of Alcohol Labels on Awareness and Knowledge of National Drinking Guidelines: A Real-World Study in Yukon, Canada. Journal of Studies on Alcohol and Drugs, 81(2), 262–272. https://doi.org/10.15288/jsad.2020.81.262
122. Zhao, J., Stockwell, T., Vallance, K., & Hobin, E. (2020). The Effects of Alcohol Warning Labels on Population Alcohol Consumption: An Interrupted Time Series Analysis of Alcohol Sales in Yukon, Canada. Journal of Studies on Alcohol and Drugs, 81(2), 225–237. https://doi.org/10.15288/jsad.2020.81.225
123. Shield, K., Manthey, J., Rylett, M., Probst, C., Wettlaufer, A., Parry, C. D. H., & Rehm, J. (2020). National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: A comparative risk assessment study. The Lancet. Public Health, 5(1), e51–e61. https://doi.org/10.1016/S2468-2667(19)30231-2
124. Australian Government Department of Health and Aged Care. (2020, December 7). Australian Alcohol Guidelines revised [Text]. Australian Alcohol Guidelines Revised; Australian Government Department of Health and Aged Care. https://www.health.gov.au/news/australian-alcohol-guidelines-revised
125. UK Chief Medical Officers’ Low Risk Drinking Guidelines (p. 11). (2016). UK Department of Health. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/545937/UK_CMOs__report.pdf
126. Paradis, C., Butt, P., Shield, K., Poole, N., Wells, T., Naimi, T., Sherk, A., & the Low-Risk Alcohol Drinking Guidelines Scientific Expert Panels. (2022). Update of Canada’s Low-Risk Alcohol Drinking Guidelines: Final Report for Public Consultation (p. 65). Canadian Centre on Substance Use and Addiction.
127. Advies in kort Richtllijnen goede voeding 2015. (2015). Gezondheidsraad Health Council of the Netherlands. https://www.gezondheidsraad.nl/binaries/gezondheidsraad/documenten/adviezen/2015/11/04/richtlijnen-goede-voeding-2015/Advies+in+kort+RGV2015.pdf
128. New advice Dutch Health Council: ‘Don’t drink alcohol or drink no more than one glass daily’. (2015, November 5). https://www.stap.nl/en/news/news.html/3531/4441/new-advice-dutch-health-council-dont-drink-alcohol-or-drink-no-more-than-one-glass-daily
129. Sundhedsstyrelsens udmeldinger om alkohol. (2022, September 19). https://www.sst.dk/da/viden/forebyggelse/alkohol/alkoholforebyggelse/sundhedsstyrelsens-udmeldinger-om-alkohol
130. Facts about moderate drinking | CDC. (2022, July 25). https://www.cdc.gov/alcohol/fact-sheets/moderate-drinking.htm
131. Wood, A. M., Kaptoge, S., Butterworth, A. S., Willeit, P., Warnakula, S., Bolton, T., Paige, E., Paul, D. S., Sweeting, M., Burgess, S., Bell, S., Astle, W., Stevens, D., Koulman, A., Selmer, R. M., Verschuren, W. M. M., Sato, S., Njølstad, I., Woodward, M., … Danesh, J. (2018). Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. The Lancet, 391(10129), 1513–1523. https://doi.org/10.1016/S0140-6736(18)30134-X
132. Socialstyrelsen. (2018). Nationella riktlinjer för prevention och behandling vid ohälsosamma levnadsvanor: Stöd för styrning och ledning. Socialstyrelsen. https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/nationella-riktlinjer/2018-6-24.pdf
133. Visseren, F. L. J., Mach, F., Smulders, Y. M., Carballo, D., Koskinas, K. C., Bäck, M., Benetos, A., Biffi, A., Boavida, J.-M., Capodanno, D., Cosyns, B., Crawford, C., Davos, C. H., Desormais, I., Di Angelantonio, E., Franco, O. H., Halvorsen, S., Hobbs, F. D. R., Hollander, M., … Williams, B. (2022). 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. European Journal of Preventive Cardiology, 29(1), 5–115. https://doi.org/10.1093/eurjpc/zwab154
134. Unger, T., Borghi, C., Charchar, F., Khan, N. A., Poulter, N. R., Prabhakaran, D., Ramirez, A., Schlaich, M., Stergiou, G. S., Tomaszewski, M., Wainford, R. D., Williams, B., & Schutte, A. E. (2020). 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension, 75(6), 1334–1357. https://doi.org/10.1161/HYPERTENSIONAHA.120.15026
135. Babor, T. F., McRee, B. G., Kassebaum, P. A., Grimaldi, P. L., Ahmed, K., & Bray, J. (2007). Screening, Brief Intervention, and Referral to Treatment (SBIRT): Toward a public health approach to the management of substance abuse. Substance Abuse, 28(3), 7–30. https://doi.org/10.1300/J465v28n03_03
136. Kaner, E. F., Beyer, F. R., Muirhead, C., Campbell, F., Pienaar, E. D., Bertholet, N., Daeppen, J. B., Saunders, J. B., & Burnand, B. (2018). Effectiveness of brief alcohol interventions in primary care populations. The Cochrane Database of Systematic Reviews, 2(2), CD004148. https://doi.org/10.1002/14651858.CD004148.pub4
137. Saitz, R. (2015). ‘SBIRT’ is the answer? Probably not. Addiction (Abingdon, England), 110(9), 1416–1417. https://doi.org/10.1111/add.12986
138. McCambridge, J. (2021). Reimagining brief interventions for alcohol: Towards a paradigm fit for the twenty first century? : INEBRIA Nick Heather Lecture 2019: This lecture celebrates the work of Nick Heather in leading thinking in respect of both brief interventions and wider alcohol sciences. Addiction Science & Clinical Practice, 16(1), 41. https://doi.org/10.1186/s13722-021-00250-w
139. Reinholdz, H., Fornazar, R., Bendtsen, P., & Spak, F. (2013). Comparison of systematic versus targeted screening for detection of risky drinking in primary care. Alcohol and Alcoholism (Oxford, Oxfordshire), 48(2), 172–179. https://doi.org/10.1093/alcalc/ags137
140. Glass, J. E., Hamilton, A. M., Powell, B. J., Perron, B. E., Brown, R. T., & Ilgen, M. A. (2015). Specialty substance use disorder services following brief alcohol intervention: A meta-analysis of randomized controlled trials. Addiction (Abingdon, England), 110(9), 1404–1415. https://doi.org/10.1111/add.12950
141. Wallhed Finn, S., Bakshi, A.-S., & Andréasson, S. (2014). Alcohol consumption, dependence, and treatment barriers: Perceptions among nontreatment seekers with alcohol dependence. Substance Use & Misuse, 49(6), 762–769. https://doi.org/10.3109/10826084.2014.891616
142. Andréasson, S., Danielsson, A.-K., & Hallgren, M. (2013). Severity of alcohol dependence in the Swedish adult population: Association with consumption and social factors. Alcohol (Fayetteville, N.Y.), 47(1), 21–25. https://doi.org/10.1016/j.alcohol.2012.10.001
143. Wallhed Finn, S. (2018). Fler kan få beroendebehandling om den integreras i primärvård. Lakartidningen, 115, E67P.
144. Holmes, J., Beard, E., Brown, J., Brennan, A., Meier, P. S., Michie, S., Stevely, A. K., Webster, L., & Buykx, P. F. (2020). Effects on alcohol consumption of announcing and implementing revised UK low-risk drinking guidelines: Findings from an interrupted time series analysis. Journal of Epidemiology and Community Health, 74(11), 942–949. https://doi.org/10.1136/jech-2020-213820
145. Babor, T. F., Casswell, S., Graham, K., Huckle, T., Livingston, M., Rehm, J., Room, R., Rossow, I., & Sornpaisarn, B. (2022). Alcohol: No Ordinary Commodity-a summary of the third edition. Addiction (Abingdon, England), 117(12), 3024–3036. https://doi.org/10.1111/add.16003
146. Kehoe, T., Gmel, G., Shield, K. D., Gmel, G., & Rehm, J. (2012). Determining the best population-level alcohol consumption model and its impact on estimates of alcohol-attributable harms. Population Health Metrics, 10, 6. https://doi.org/10.1186/1478-7954-10-6
147. Rossow, I., & Mäkelä, P. (2021). Public Health Thinking Around Alcohol-Related Harm: Why Does Per Capita Consumption Matter? Journal of Studies on Alcohol and Drugs, 82(1), 9–17.
148. Razvodovsky, Y. E. (2014). Contribution of alcohol to hypertension mortality in Russia. Journal of Addiction, 2014, 483910. https://doi.org/10.1155/2014/483910
149. Norström, T. (2011). The role of alcohol in the Russian mortality crisis. Addiction (Abingdon, England), 106(11), 1957–1965. https://doi.org/10.1111/j.1360-0443.2011.03513.x
150. Roerecke, M. (2021). Alcohol’s Impact on the Cardiovascular System. Nutrients, 13(10), 3419. https://doi.org/10.3390/nu13103419
151. Day, E., & Rudd, J. H. F. (2019). Alcohol use disorders and the heart. Addiction (Abingdon, England), 114(9), 1670–1678. https://doi.org/10.1111/add.14703
152. Gonzaga, N. A., do Vale, G. T., Parente, J. M., Yokota, R., De Martinis, B. S., Casarini, D. E., Castro, M. M., & Tirapelli, C. R. (2018). Ethanol withdrawal increases blood pressure and vascular oxidative stress: A role for angiotensin type 1 receptors. Journal of the American Society of Hypertension: JASH, 12(7), 561–573. https://doi.org/10.1016/j.jash.2018.03.012
153. Leal, S., Ricardo Jorge, D.-O., Joana, B., Maria, S. S., & Isabel, S. S. (2017). Heavy Alcohol Consumption Effects on Blood Pressure and on Kidney Structure Persist After Long-Term Withdrawal. Kidney & Blood Pressure Research, 42(4), 664–675. https://doi.org/10.1159/000482022
154. Chalmers, J., Arima, H., Harrap, S., Touyz, R. M., & Park, J. B. (2013). Global survey of current practice in management of hypertension as reported by societies affiliated with the International Society of Hypertension. Journal of Hypertension, 31(5), 1043–1048. https://doi.org/10.1097/HJH.0b013e32835f7eef
155. Stamler, R. (1991). Implications of the INTERSALT study. Hypertension (Dallas, Tex.: 1979), 17(1 Suppl), I16-20. https://doi.org/10.1161/01.hyp.17.1_suppl.i16