1. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food-10 states, 2007 (2008) MMWR Morb Mortal Wkly Rep 57, 366–70.
2. Listeria Available at: https://www.medscinet.se/infpreg/healthcareinfoMore.aspx?topic=23 [Accessed October 16, 2019].
3. Listeria monocytogenes Available at: https://www.livsmedelsverket.se/livsmedel-och-innehall/bakterier-virus-parasiter-och-mogelsvampar1/bakterier/listeria-monocytogenes/ [Accessed August 23, 2019].
4. FDA Pregnancy Categories Available at: https://chemm.nlm.nih.gov/pregnancycategories.htm [Accessed September 23, 2019].
5. Janusmed amning Available at: https://janusinfo.se/beslutsstod/janusmedamning.4.72866553160e98a7ddf1cef.html [Accessed October 17, 2019].
6. Janusmed fosterpåverkan Available at: https://janusinfo.se/beslutsstod/janusmedfosterpaverkan.4.72866553160e98a7ddf1ce6.html [Accessed October 17, 2019].
7. Barker M, Dombrowski SU, Colbourn T, Fall CHD, Kriznik NM, Lawrence WT, Norris SA, Ngaiza G, et al (2018) Intervention strategies to improve nutrition and health behaviours before conception. Lancet 391, 1853–64.
8. Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, Barker M, Saffery R, et al (2018) Origins of lifetime health around the time of conception: causes and consequences. Lancet 391, 1842–52.
9. Stephenson J, Heslehurst N, Hall J, Schoenaker DAJM, Hutchinson J, Cade JE, Poston L, Barrett G, et al (2018) Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet 391, 1830–41.
10. Terasaki LS, Gomez J, Schwarz JM (2016) An examination of sex differences in the effects of early-life opiate and alcohol exposure. Philos Trans R Soc Lond, B, Biol Sci 371, 20150123.
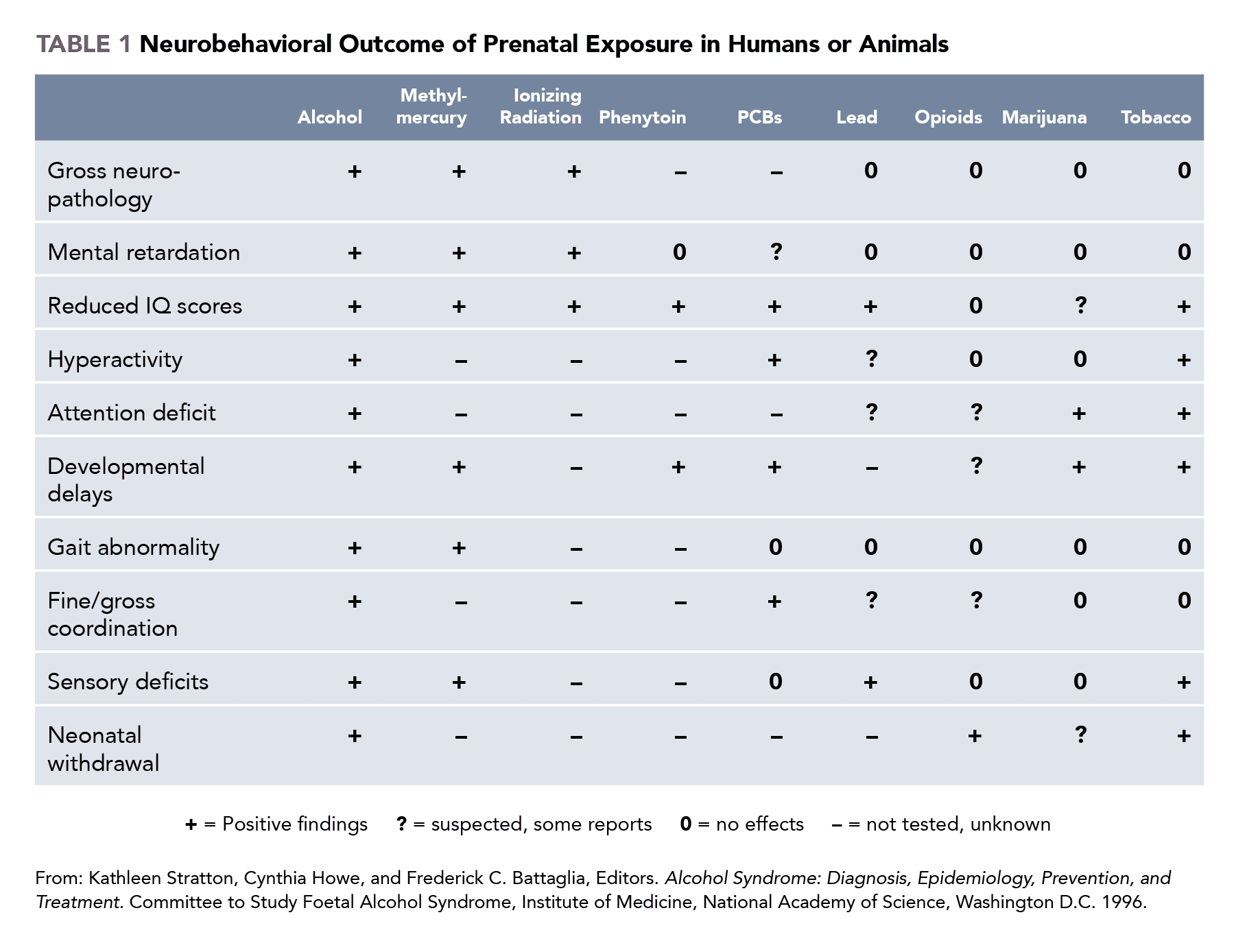
11. Stratton K, Howe C, Battaglia FC, others (1996) Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment, National Academies Press.
12. Popova S, Lange S, Probst C, Gmel G, Rehm J (2017) Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. Lancet Glob Health 5, e290–e299.
13. Chudley AE (2008) Fetal alcohol spectrum disorder: counting the invisible – mission impossible? Arch Dis Child 93, 721–2.
14. Olegård R, Sabel KG, Aronsson M, Sandin B, Johansson PR, Carlsson C, Kyllerman M, Iversen K, Hrbek A (1979) Effects on the child of alcohol abuse during pregnancy. Retrospective and prospective studies. Acta Paediatr Scand Suppl 275, 112–21.
15. Lange S, Probst C, Gmel G, Rehm J, Burd L, Popova S (2017) Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatr 171, 948–56.
16. Greenmyer JR, Klug MG, Kambeitz C, Popova S, Burd L A Multicountry Updated Assessment of the Economic Impact of Fetal Alcohol Spectrum Disorder: Costs for Children and Adults. J Addict Med 12, 466–73.
17. Ericson L, Magnusson L, Hovstadius B (2017) Societal costs of fetal alcohol syndrome in Sweden. Eur J Health Econ 18, 575–85.
18. Thanh NX, Jonsson E (2018) Total Cost of FASD Including the Economics of FASD Associated with Crimes. In: Ethical and Legal Perspectives in Fetal Alcohol Spectrum Disorders (FASD), Springer, pp 49–66.
19. Skagerström J, Häggström-Nordin E, Alehagen S (2015) The voice of non-pregnant women on alcohol consumption during pregnancy: a focus group study among women in Sweden. BMC Public Health 15, 1193.
20. Bodin M, Käll L, Tydén T, Stern J, Drevin J, Larsson M (2017) Exploring men’s pregnancy-planning behaviour and fertility knowledge:a survey among fathers in Sweden. Ups J Med Sci 122, 127–35.
21. Bailey BA, Sokol RJ (2011) Prenatal alcohol exposure and miscarriage, stillbirth, preterm delivery, and sudden infant death syndrome. Alcohol Res Health 34, 86–91.
22. McQuire C, Daniel R, Hurt L, Kemp A, Paranjothy S (2019) The causal web of foetal alcohol spectrum disorders: a review and causal diagram. Eur Child Adolesc Psychiatry. doi:10.1007/s00787-018-1264-3.
23. Rangmar J, Fahlke C (2013) Fetal alcohol spectrum disorders – Psykosociala konsekvenser av och preventiva aspekter på alkoholrelaterade fosterskador. Nka Barn som anhöriga 2013:4.
24. Välkommen till FAS-portalen Available at: https://www.fasportalen.se/ [Accessed October 17, 2019].
25. Sarman I, Rangmar J (2017) Alkohol under graviditet kan riskera folkhälsan-Alkohol under fosterlivet påverkar inte bara barnets hjärna-risk för kardiovaskulära och metabola sjukdomar på sikt. Lakartidningen 114.
26. Larcher V, Brierley J (2014) Fetal alcohol syndrome (FAS) and fetal alcohol spectrum disorder (FASD)-diagnosis and moral policing; an ethical dilemma for paediatricians. Arch Dis Child 99, 969–70.
27. Gavaghan C (2009) “You can’t handle the truth”; medical paternalism and prenatal alcohol use. J Med Ethics 35, 300–3.
28. Naimi TS, Lipscomb LE, Brewer RD, Gilbert BC (2003) Binge drinking in the preconception period and the risk of unintended pregnancy: implications for women and their children. Pediatrics 111, 1136–41.
29. Skagerstróm J, Chang G, Nilsen P (2011) Predictors
of drinking during pregnancy: a systematic review.
J Womens Health (Larchmt) 20, 901–13.
30. Tran NT, Najman JM, Hayatbakhsh R (2015) Predictors of maternal drinking trajectories before and after pregnancy: evidence from a longitudinal study. Aust N Z J Obstet Gynaecol 55, 123–30.
31. Anderson AE, Hure AJ, Forder P, Powers JR, Kay-Lambkin FJ, Loxton DJ (2013) Predictors of antenatal alcohol use among Australian women: a prospective cohort study. BJOG 120, 1366–74.
32. Mallard SR, Connor JL, Houghton LA (2013) Maternal factors associated with heavy periconceptional alcohol intake and drinking following pregnancy recognition: a post-partum survey of New Zealand women. Drug Alcohol Rev 32, 389–97.
33. Denny CH, Acero CS, Naimi TS, Kim SY (2019) Consumption of Alcohol Beverages and Binge Drinking Among Pregnant Women Aged 18-44 Years – United States, 2015-2017. MMWR Morb Mortal Wkly Rep 68, 365–8.
34. Lange S, Probst C, Rehm J, Popova S (2017) Prevalence of binge drinking during pregnancy by country and World Health Organization region: Systematic review and meta-analysis. Reprod Toxicol 73, 214–21.
35. Trolldal B (2019) Alkoholkonsumtionen i Sverige 2018. CAN Rapport 184.
36. McBride N, Johnson S (2016) Fathers’ Role in Alcohol-Exposed Pregnancies: Systematic Review of Human Studies. Am J Prev Med 51, 240–8.
37. Crane CA, Godleski SA, Przybyla SM, Schlauch RC, Testa M (2016) The Proximal Effects of Acute Alcohol Consumption on Male-to-Female Aggression: A Meta-Analytic Review of the Experimental Literature. Trauma Violence Abuse 17, 520–31.
38. Rehm J, Shield KD, Joharchi N, Shuper PA (2012) Alcohol consumption and the intention to engage in unprotected sex: systematic review and meta-analysis of experimental studies. Addiction 107, 51–9.
39. Rothman EF, McNaughton Reyes L, Johnson RM, LaValley M (2012) Does the alcohol make them do it? Dating violence perpetration and drinking among youth. Epidemiol Rev 34, 103–19.
40. Scott-Sheldon LAJ, Carey KB, Cunningham K, Johnson BT, Carey MP (2016) Alcohol Use Predicts Sexual Decision-Making: A Systematic Review and Meta-Analysis of the Experimental Literature. AIDS Behav 20 Suppl 1, S19–39.
41. Wilson IM, Eurenius E, Lindkvist M, Edin K, Edvardsson K (2019) Is there an association between pregnant women’s experience of violence and their partner’s drinking? A Swedish population-based study. Midwifery 69, 84–91.
42. Bianchi E, Boekelheide K, Sigman M, Braun JM, Eliot M, Hall SJ, Dere E, Hwang K (2019) Spermatozoal large RNA content is associated with semen characteristics, sociodemographic and lifestyle factors. PLoS ONE 14, e0216584.
43. Li Y, Lin H, Li Y, Cao J (2011) Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril 95, 116–23.
44. Borges E, Braga DP de AF, Provenza RR, Figueira R de CS, Iaconelli A, Setti AS (2018) Paternal lifestyle factors in relation to semen quality and in vitro reproductive outcomes. Andrologia 50, e13090.
45. Milne E, Greenop KR, Scott RJ, de Klerk NH, Bower C, Ashton LJ, Heath JA, Armstrong BK (2013) Parental alcohol consumption and risk of childhood acute lymphoblastic leukemia and brain tumors. Cancer Causes Control 24, 391–402.
46. Zhang S, Wang L, Yang T, Chen L, Zhao L, Wang T, Chen L, Ye Z, et al (2019) Parental alcohol consumption and the risk of congenital heart diseases in offspring: An updated systematic review and meta-analysis. Eur J Prev Cardiol, 2047487319874530.
47. Steinberger EK, Ferencz C, Loffredo CA (2002) Infants with single ventricle: a population-based epidemiological study. Teratology 65, 106–15.
48. Landberg J, Danielsson A-K, Falkstedt D, Hemmingsson T (2018) Fathers’ Alcohol Consumption and Long-Term Risk for Mortality in Offspring. Alcohol Alcohol 53, 753–9.
49. Skagerström J, Alehagen S, Häggström-Nordin E, Årestedt K, Nilsen P (2013) Prevalence of alcohol use before and during pregnancy and predictors of drinking during pregnancy: a cross sectional study in Sweden. BMC Public Health 13, 780.
50. Alati R, Davey Smith G, Lewis SJ, Sayal K, Draper ES, Golding J, Fraser R, Gray R (2013) Effect of prenatal alcohol exposure on childhood academic outcomes: contrasting maternal and paternal associations in the ALSPAC study. PLoS ONE 8, e74844.
51. Kalisch-Smith JI, Moritz KM (2017) Detrimental effects of alcohol exposure around conception: putative mechanisms. Biochemistry and Cell Biology 96, 107–16.
52. Kesmodel US, Nygaard SS, Mortensen EL, Bertrand J, Denny CH, Glidewell A, Astley Hemingway S (2019) Are Low-to-Moderate Average Alcohol Consumption and Isolated Episodes of Binge Drinking in Early Pregnancy Associated with Facial Features Related to Fetal Alcohol Syndrome in 5-Year-Old Children? Alcohol Clin Exp Res 43, 1199–212.
53. Flak AL, Su S, Bertrand J, Denny CH, Kesmodel US, Cogswell ME (2014) The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: a meta-analysis. Alcohol Clin Exp Res 38, 214–26.
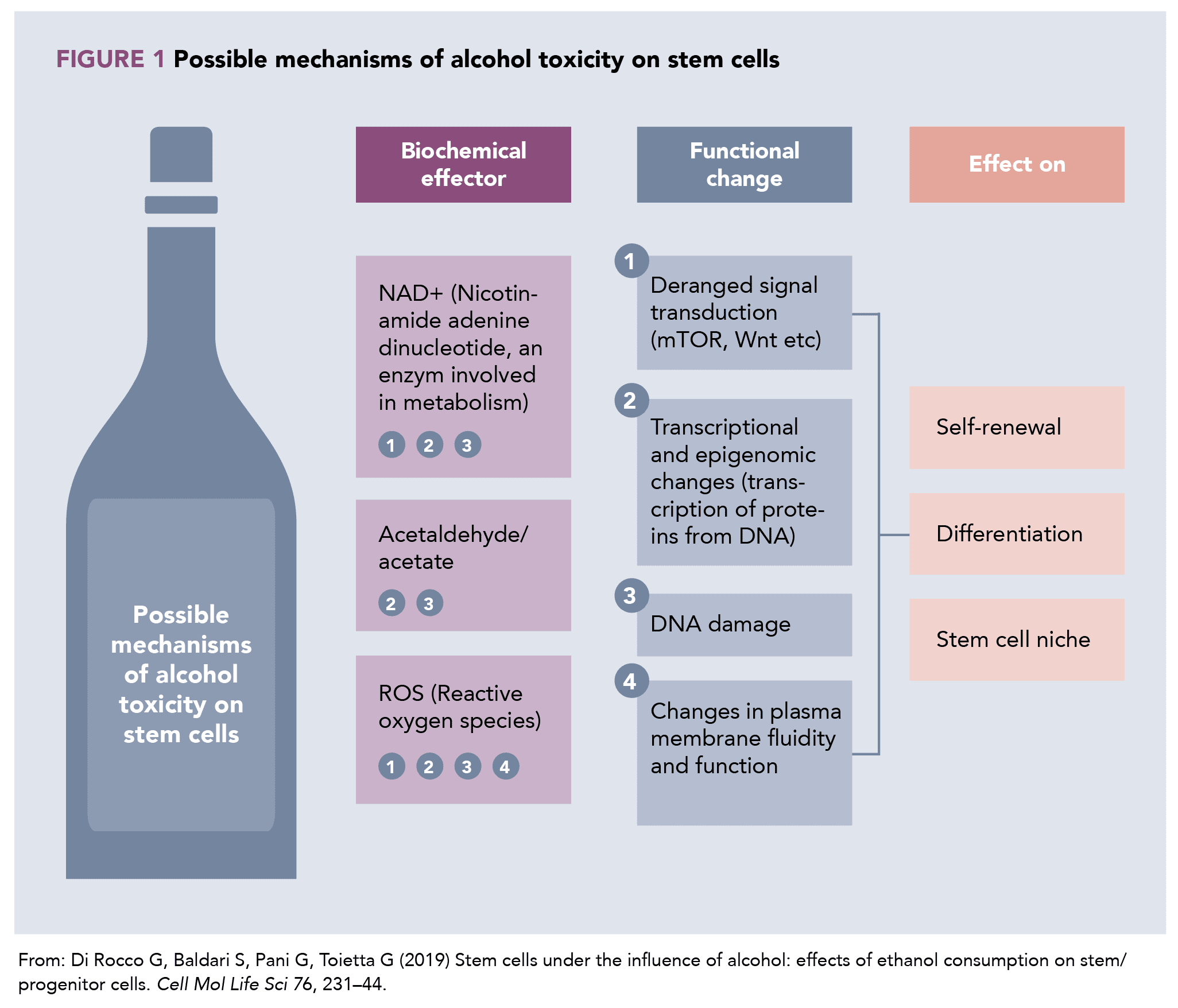
54. Di Rocco G, Baldari S, Pani G, Toietta G (2019) Stem cells under the influence of alcohol: effects of ethanol consumption on stem/progenitor cells. Cell Mol Life Sci 76, 231–44.
55. Odendaal HJ, Steyn DW, Elliott A, Burd L (2009) Combined effects of cigarette smoking and alcohol consumption on perinatal outcome. Gynecol Obstet Invest 67, 1–8.
56. Bailey BA, Sokol RJ (2008) Is prematurity a part of fetal alcohol spectrum disorder? Expert Review of Obstetrics & Gynecology 3, 245–55.
57. Gauthier TW (2015) Prenatal alcohol exposure and the developing immune system. Alcohol research: current reviews 37, 279.
58. Patra J, Bakker R, Irving H, Jaddoe VWV, Malini S, Rehm J (2011) Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG 118, 1411–21.
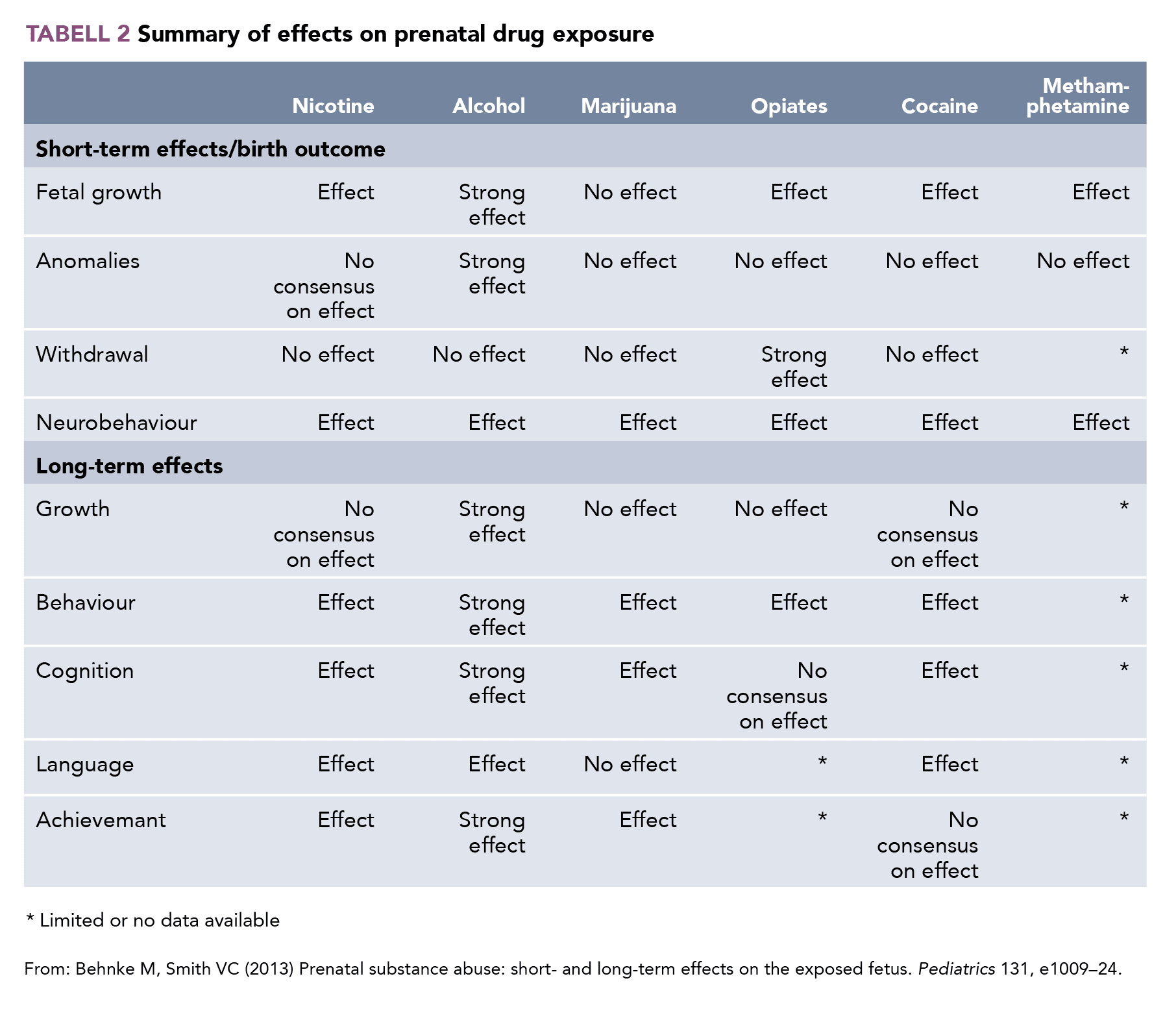
59. Behnke M, Smith VC (2013) Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics 131, e1009–24.
60. Andréasson S, Chikritzhs T, Dangardt F, Holder H, Naimi T, Stockwell T (2017) Alkohol Och Våld – En Översikt Av Internationell Och Svensk Forskning, CERA, Göteborgs Universitet, IOGT-NTO, Svenska Läkaresällskapet.
61. WHO (2006) Interpersonal Violence and Alcohol. WHO Policy Briefing, Geneva: World Health Organisation.
62. Boles SM, Miotto K (2003) Substance abuse and violence: A review of the literature. Aggression and violent behavior 8, 155–74.
63. WHO (2009) Preventing Violence by Reducing the Availability and Harmful Use of Alcohol, Geneva: World Health Organisation.
64. James L, Brody D, Hamilton Z (2013) Risk factors for domestic violence during pregnancy: a meta-analytic review. Violence Vict 28, 359–80.
65. Heller M, Burd L (2014) Review of ethanol dispersion, distribution, and elimination from the fetal compartment. Birth Defects Res Part A Clin Mol Teratol 100, 277–83.
66. Gupta KK, Gupta VK, Shirasaka T (2016) An Update on Fetal Alcohol Syndrome-Pathogenesis, Risks, and Treatment. Alcohol Clin Exp Res 40, 1594–602.
67. Pikkarainen PH, Räihä NC (1967) Development of alcohol dehydrogenase activity in the human liver. Pediatr Res 1, 165–8.
68. Caputo C, Wood E, Jabbour L (2016) Impact of fetal alcohol exposure on body systems: A systematic review. Birth Defects Res C Embryo Today 108, 174–80.
69. Popova S, Lange S, Shield K, Mihic A, Chudley AE, Mukherjee RAS, Bekmuradov D, Rehm J (2016) Comorbidity of fetal alcohol spectrum disorder: a systematic review and meta-analysis. Lancet 387, 978–87.
70. Astley-Hemingway S, Bledsoe J, Davies J, Brooks A, Jirikowic T, Olson E, Thorne J (2019) Twin study confirms virtually identical prenatal alcohol exposures can lead to markedly different fetal alcohol spectrum disorder outcomes- fetal genetics influences fetal vulnerability. Advances in Pediatric Research 5, 1–19.
71. Abbott CW, Rohac DJ, Bottom RT, Patadia S, Huffman KJ (2018) Prenatal Ethanol Exposure and Neocortical Development: A Transgenerational Model of FASD. Cereb Cortex 28, 2908–21.
72. Akison LK, Moritz KM, Reid N (2019) Adverse reproductive outcomes associated with fetal alcohol exposure: a systematic review. Reproduction 157, 329–43.
73. Ramlau-Hansen CH, Toft G, Jensen MS, Strandberg-Larsen K, Hansen ML, Olsen J (2010) Maternal alcohol consumption during pregnancy and semen quality in the male offspring: two decades of follow-up. Hum Reprod 25, 2340–5.
74. VandeVoort CA, Grimsrud KN, Midic U, Mtango N, Latham KE (2015) Transgenerational effects of binge drinking in a primate model: implications for human health. Fertil Steril 103, 560–9.
75. Lecuyer M, Laquerrière A, Bekri S, Lesueur C, Ramdani Y, Jégou S, Uguen A, Marcorelles P, et al (2017) PLGF, a placental marker of fetal brain defects after in utero alcohol exposure. Acta Neuropathol Commun 5, 44.
76. Nunez CC, Roussotte F, Sowell ER (2011) Focus on: structural and functional brain abnormalities in fetal alcohol spectrum disorders. Alcohol Res Health 34, 121–31.
77. Karalexi MA, Dessypris N, Thomopoulos TP, Ntouvelis E, Kantzanou M, Diamantaras A-A, Moschovi M, Baka M, et al (2017) Parental alcohol consumption and risk of leukemia in the offspring: a systematic review and meta-analysis. Eur J Cancer Prev 26, 433–41.
78. Latino-Martel P, Chan DSM, Druesne-Pecollo N, Barrandon E, Hercberg S, Norat T (2010) Maternal alcohol consumption during pregnancy and risk of childhood leukemia: systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 19, 1238–60.
79. Orsi L, Rudant J, Ajrouche R, Leverger G, Baruchel A, Nelken B, Pasquet M, Michel G, et al (2015) Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy, and childhood acute leukemia: the ESTELLE study. Cancer Causes Control 26, 1003–17.
80. O’Neil, Erica, (2011). Developmental Timeline of Alcohol-Induced Birth Defects. Embryo Project Encyclopedia. ISSN: 1940-5030 http://embryo.asu.edu/handle/10776/2101.
81 Dolk H, Loane M, Garne E (2011) Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation 123, 841–9.
82. Henderson J, Gray R, Brocklehurst P (2007) Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome. BJOG 114, 243–52.
83. Yang J, Qiu H, Qu P, Zhang R, Zeng L, Yan H (2015) Prenatal Alcohol Exposure and Congenital Heart Defects: A Meta-Analysis. PLoS ONE 10, e0130681.
84. Hoffman JI, Kaplan S (2002) The incidence of congenital heart disease. Journal of the American College of Cardiology 39, 1890–900.
85. Giliberti D, Mohan SS, Brown LAS, Gauthier TW (2013) Perinatal exposure to alcohol: implications for lung development and disease. Paediatr Respir Rev 14, 17–21.
86. Gauthier TW, Brown LAS (2017) In utero alcohol effects on foetal, neonatal and childhood lung disease. Paediatr Respir Rev 21, 34–7.
87. Hofer R, Burd L (2009) Review of published studies of kidney, liver, and gastrointestinal birth defects in fetal alcohol spectrum disorders. Birth Defects Res Part A Clin Mol Teratol 85, 179–83.
88. Qazi Q, Masakawa A, Milman D, McGann B, Chua A, Haller J (1979) Renal anomalies in fetal alcohol syndrome. Pediatrics 63, 886–9.
89. Assadi F (2014) Renal dysfunction in fetal alcohol syndrome: a potential contributor on developmental disabilities of offspring. J Renal Inj Prev 3, 83–6.
90. Assadi FK, Zajac CS (1992) Ultrastructural changes in the rat kidney following fetal exposure to ethanol. Alcohol 9, 509–12.
91. Liu Q, Gao F, Liu X, Li J, Wang Y, Han J, Wang X (2016) Prenatal alcohol exposure and offspring liver dysfunction: a systematic review and meta-analysis. Arch Gynecol Obstet 294, 225–31.
92. Shen L, Liu Z, Gong J, Zhang L, Wang L, Magdalou J, Chen L, Wang H (2014) Prenatal ethanol exposure programs an increased susceptibility of non-alcoholic fatty liver disease in female adult offspring rats. Toxicol Appl Pharmacol 274, 263–73.
93. Uc A, Vasiliauskas E, Piccoli DA, Flores AF, Di Lorenzo C, Hyman PE (1997) Chronic intestinal pseudoobstruction associated with fetal alcohol syndrome. Dig Dis Sci 42, 1163–7.
94. Noor S, Milligan ED (2018) Lifelong Impacts of Moderate Prenatal Alcohol Exposure on Neuroimmune Function. Front Immunol 9, 1107.
95. Comasco E, Rangmar J, Eriksson UJ, Oreland L (2018) Neurological and neuropsychological effects of low and moderate prenatal alcohol exposure. Acta Physiol (Oxf) 222. doi:10.1111/apha.12892.
96. McCormack C, Hutchinson D, Burns L, Youssef G, Wilson J, Elliott E, Allsop S, Najman J, et al (2018) Maternal and partner prenatal alcohol use and infant cognitive development. Drug Alcohol Depend 185, 330–8.
97. Subramoney S, Eastman E, Adnams C, Stein DJ, Donald KA (2018) The Early Developmental Outcomes of Prenatal Alcohol Exposure: A Review. Front Neurol 9, 1108.
98. Light drinking during pregnancy does not harm baby: study Available at: https://nypost.com/2017/09/11/light-drinking-during-pregnancy-does-not-harm-baby-study/ [Accessed August 21, 2019].
99. Alkohol og graviditet – Ok eller farligt? Available at: https://graviditet.dk/alkohol-graviditet [Accessed August 22, 2019].
100. Agnes Wold: ”Några glas alkohol är inte farligt för fostret – det är bara moralism” Available at: https://www.baaam.se/agnes-wold-nagra-glas-alkohol-ar-inte-farligt-for-fostret-det-ar-bara-moralism [Accessed August 22, 2019].
101. Gray R, Henderson J (2006) Review of the Fetal Effects of Prenatal Alcohol Exposure, Oxford: National Perinatal Epidemiology Unit, University of Oxford Available at: https://www.npeu.ox.ac.uk/downloads/files/reports/Alcohol-in-Pregnancy-Report.pdf.
102. Mamluk L, Edwards HB, Savović J, Leach V, Jones T, Moore THM, Ijaz S, Lewis SJ, et al (2017) Low alcohol consumption and pregnancy and childhood outcomes: time to change guidelines indicating apparently “safe” levels of alcohol during pregnancy? A systematic review and meta-analyses. BMJ Open 7, e015410.
103. Easey KE, Dyer ML, Timpson NJ, Munafò MR (2019) Prenatal alcohol exposure and offspring mental health: A systematic review. Drug Alcohol Depend 197, 344–53.
104. Hinke Kessler Scholder S, Wehby GL, Lewis S, Zuccolo L (2014) Alcohol exposure in utero and child academic achievement. The Economic Journal 124, 634–67.
105. Lewis SJ, Zuccolo L, Davey Smith G, Macleod J, Rodriguez S, Draper ES, Barrow M, Alati R, et al (2012) Fetal alcohol exposure and IQ at age 8: evidence from a population-based birth-cohort study. PLoS ONE 7, e49407.
106. Murray J, Burgess S, Zuccolo L, Hickman M, Gray R, Lewis SJ (2016) Moderate alcohol drinking in pregnancy increases risk for children’s persistent conduct problems: causal effects in a Mendelian randomisation study. J Child Psychol Psychiatry 57, 575–84.
107. Zuccolo L, Lewis SJ, Smith GD, Sayal K, Draper ES, Fraser R, Barrow M, Alati R, et al (2013) Prenatal alcohol exposure and offspring cognition and school performance. A “Mendelian randomization” natural experiment. Int J Epidemiol 42, 1358–70.
108. Schambra UB, Lewis CN, Harrison TA (2017) Deficits in spatial learning and memory in adult mice following acute, low or moderate levels of prenatal ethanol exposure during gastrulation or neurulation. Neurotoxicol Teratol 62, 42–54.
109. Abate P, Pueta M, Spear NE, Molina JC (2008) Fetal learning about ethanol and later ethanol responsiveness: evidence against “safe” amounts of prenatal exposure. Exp Biol Med (Maywood) 233, 139–54.
110. Babor T, Caetano R, Casswell S, Edwards G, Giesbrecht N, Graham K, Grube J, Hill L, et al (2010) Alcohol: No Ordinary Commodity: Research and Public Policy 2nd Ed, New York (NY): Oxford University Press.
111. WHO (2018) Global Status Report on Alcohol and Health 2018, Geneva: World Health Organization.
112. Silver D, Macinko J, Giorgio M, Bae JY (2019) Evaluating the relationship between binge drinking rates and a replicable measure of U.S. state alcohol policy environments. PLoS ONE 14, e0218718.
113. Zhang N (2010) Alcohol taxes and birth outcomes. Int J Environ Res Public Health 7, 1901–12.
114. Campbell CA, Hahn RA, Elder R, Brewer R, Chattopadhyay S, Fielding J, Naimi TS, Toomey T, et al (2009) The effectiveness of limiting alcohol outlet density as a means of reducing excessive alcohol consumption and alcohol-related harms. Am J Prev Med 37, 556–69.
115. Jans J (2017) Causes and Consequences of Early-life Conditions: Alcohol, Pollution and Parental Leave Policies.
116. Nilsson J (2017) Alcohol Availability, Prenatal Conditions, and Long-Term Economic Outcomes. Journal of Political Economy 125, 1149–207.
117. Seabrook JA, Woods N, Clark A, de Vrijer B, Penava D, Gilliland J (2018) The association between alcohol outlet accessibility and adverse birth outcomes: A retrospective cohort study. J Neonatal Perinatal Med 11, 71–7.
118. Schölin L (2016) Prevention of Harm Caused by Alcohol Exposure in Pregnancy – Rapid Review and Case Studies from Member States, WHO, Copenhagen: World Health Organization Regional Office of for Europe.
119. Fergie L, Campbell KA, Coleman-Haynes T, Ussher M, Cooper S, Coleman T (2019) Identifying Effective Behavior Change Techniques for Alcohol and Illicit Substance Use During Pregnancy: A Systematic Review. Ann Behav Med 53, 769–81.
120. Grönqvist E, Norén A, Sjögren A, Svaleryd H (2016) Sober Mom, Healthy Baby? Effects of Brief Alcohol Interventions in Swedish Maternity Care, Uppsala: Institute for Evaluation of Labour Market and Education Policy (IFAU).
121. Grönqvist E, Norén A, Sjögren A, Svaleryd H (2016) Nyktrare Mammor, Friskare Barn? Effekter Av AUDIT-Screening Och Motiverande Samtal I Svensk Mödravård, Uppsala: Institutet för arbetsmarknads- och utbildningspolitisk utvärdering (IFAU).
122. Högberg H, Spak F, Larsson M (2015) Dialogue between midwives and parents-to-be about alcohol, from a life cycle perspective—an intervention study. Creative Education 6, 489–500.
123. Hankin JR, Firestone IJ, Sloan JJ, Ager JW, Sokol RJ, Martier SS (1996) Heeding the alcoholic beverage warning label during pregnancy: multiparae versus nulliparae. J Stud Alcohol 57, 171–7.
124. Roberts SCM, Mericle AA, Subbaraman MS, Thomas S, Treffers RD, Delucchi KL, Kerr WC (2019) State Policies Targeting Alcohol Use during Pregnancy and Alcohol Use among Pregnant Women 1985-2016: Evidence from the Behavioral Risk Factor Surveillance System. Womens Health Issues 29, 213–21.
125. Subbaraman MS, Thomas S, Treffers R, Delucchi K, Kerr WC, Martinez P, Roberts SCM (2018) Associations Between State-Level Policies Regarding Alcohol Use Among Pregnant Women, Adverse Birth Outcomes, and Prenatal Care Utilization: Results from 1972 to 2013 Vital Statistics. Alcohol Clin Exp Res. doi:10.1111/acer.13804.
126. Basics about FASDs Available at: https://www.cdc.gov/ncbddd/fasd/facts.html [Accessed July 17, 2019].
127. Breastfeeding Available at: https://www.who.int/nutrition/topics/exclusive_breastfeeding/en/ [Accessed August 22, 2019].
128. Horta B, Victora C (2013) Long-Term Effects of Breastfeeding: A Systematic Review, Geneva: World Health Organization.
129. Horta BL, Loret de Mola C, Victora CG (2015) Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr 104, 14–9.
130. Tearne E, Cox K, Giglia R (2017) Patterns of Alcohol Intake of Pregnant and Lactating Women in Rural Western Australia. Matern Child Health J 21, 2068–77.
131. Wilson J, Tay RY, McCormack C, Allsop S, Najman J, Burns L, Olsson CA, Elliott E, et al (2017) Alcohol consumption by breastfeeding mothers: Frequency, correlates and infant outcomes. Drug Alcohol Rev 36, 667–76.
132. Giglia RC (2010) Alcohol and lactation: An updated systematic review. Nutrition & Dietetics 67, 237–43.
133. Haastrup MB, Pottegård A, Damkier P (2014) Alcohol and breastfeeding. Basic Clin Pharmacol Toxicol 114, 168–73.
134. Giglia R, Binns C (2006) Alcohol and lactation: a systematic review. Nutrition & Dietetics 63, 103–16.
135. Alvik A, Haldorsen T, Lindemann R (2006) Alcohol consumption, smoking and breastfeeding in the first six months after delivery. Acta Paediatr 95, 686–93.
136. Lanting CI, van Dommelen P, van der Pal-de Bruin KM, Bennebroek Gravenhorst J, van Wouwe JP (2015) Prevalence and pattern of alcohol consumption during pregnancy in the Netherlands. BMC Public Health 15, 723.
137. Lawton ME (1985) Alcohol in breast milk. Aust N Z J Obstet Gynaecol 25, 71–3.
138. Kesäniemi YA (1974) Ethanol and acetaldehyde in the milk and peripheral blood of lactating women after ethanol administration. J Obstet Gynaecol Br Commonw 81, 84–6.
139. Mennella JA, Beauchamp GK (1993) Beer, breast feeding, and folklore. Dev Psychobiol 26, 459–66.
140. Mennella JA, Beauchamp GK (1991) The transfer of alcohol to human milk. Effects on flavor and the infant’s behavior. N Engl J Med 325, 981–5.
141. Mennella JA, Pepino MY, Teff KL (2005) Acute alcohol consumption disrupts the hormonal milieu of lactating women. J Clin Endocrinol Metab 90, 1979–85.
142. Schuetze P, Eiden RD, Chan AW (2002) The Effects of Alcohol in Breast Milk on Infant Behavioral State and Mother-Infant Feeding Interactions. Infancy 3, 349–63.
143. Little RE, Lambert MD, Worthington-Roberts B (1990) Drinking and smoking at 3 months postpartum by lactation history. Paediatr Perinat Epidemiol 4, 290–302.
144. Little RE, Northstone K, Golding J (2002) Alcohol, breastfeeding, and development at 18 months. Pediatrics 109, E72–2.
145. Gibson L, Porter M (2018) Drinking or Smoking While Breastfeeding and Later Cognition in Children. Pediatrics 142. doi:10.1542/peds.2017-4266.
146. Mennella JA, Gerrish CJ (1998) Effects of exposure to alcohol in mother’s milk on infant sleep. Pediatrics 101, E2.
147. Backstrand JR, Goodman AH, Allen LH, Pelto GH (2004) Pulque intake during pregnancy and lactation in rural Mexico: alcohol and child growth from 1 to 57 months. Eur J Clin Nutr 58, 1626–34.
148. Csaba G, Kovács P, Pállinger É (2006) Changes in the endorphin and serotonin content of rat immune cells during adulthood following maternal exposure to ethanol during pregnancy and lactation. Alcohol 38, 111–6.
149. Hekmatpanah J, Haghighat N, Adams CR (1994) Alcohol consumption by nursing rats and its effect on the cerebellum of the offspring. Alcohol Alcohol 29, 535–47.
150. Van Nguyen JM, Abenhaim HA (2013) Sudden infant death syndrome: review for the obstetric care provider. Am J Perinatol 30, 703–14.
151. Phillips DP, Brewer KM, Wadensweiler P (2011) Alcohol as a risk factor for sudden infant death syndrome (SIDS). Addiction 106, 516–25.
152. Blair PS, Sidebotham P, Pease A, Fleming PJ (2014) Bed-sharing in the absence of hazardous circumstances: is there a risk of sudden infant death syndrome? An analysis from two case-control studies conducted in the UK. PLoS ONE 9, e107799.
153. O’Leary CM, Jacoby PJ, Bartu A, D’Antoine H, Bower C (2013) Maternal alcohol use and sudden infant death syndrome and infant mortality excluding SIDS. Pediatrics 131, e770–8.
154. Ammande Available at: https://www.livsmedelsverket.se/matvanor-halsa-miljo/kostrad-och-matvanor/ammande?AspxAutoDetectCookieSupport=1 [Accessed August 22, 2019].
155. Råd til kvinner som ammer: Alkohol Available at: https://www.helsedirektoratet.no/faglige-rad/rad-til-kvinner-som-ammer/alkohol [Accessed August 22, 2019].
156. Centro nazionale di epidemiologia, sorveglianza e promozione della salute Available at: https://www.epicentro.iss.it/ [Accessed August 22, 2019].
157. Kein Alkohol in der Stillzeit Available at: https://www.kenn-dein-limit.de/alkohol/schwangerschaft-und-stillzeit/stillzeit/ [Accessed August 22, 2019].
158. Alkohol, Rauchen und Medikamente – Gesund Leben in der Stillzeit Available at: https://www.gesund-ins-leben.de/inhalt/alkohol-rauchen-29429.html [Accessed August 22, 2019].
159. Puis-je boire alors que j’allaite mon enfant ? Available at: https://www.alcool-info-service.fr/alcool-et-vous/alcool-grossesse/allaitement-alcool [Accessed August 22, 2019].
160. CCSA (2018) Canada’s Low-Risk Alcohol Drinking Guidelines. Available at: https://ccsa.ca/sites/default/files/2019-09/2012-Canada-Low-Risk-Alcohol-Drinking-Guidelines-Brochure-en.pdf.
161. UK Chief Medical Officers Low Risk Drinking Guidelines (2016) London: Department of Health Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/545937/UK_CMOs__report.pdf.
162. NHMRC (2009) Australian Guidelines to Reduce Health Risks from Drinking Alcohol, Canberra: Commonwealth of Australia Available at: https://www.nhmrc.gov.au/about-us/publications/australian-guidelines-reduce-health-risks-drinking-alcohol.
163. Allebeck P, Andreasson S, Wåhlin S, Ramstedt M, Gripenberg J, Damström-Thakker K, Heinemans N (2018) Alkoholkonsumtion Och Risknivåer. Kunskapsunderlag Och Förslag till Rekommendationer, Stockholm: Centrum för epidemiologi och samhällsmedicin, Stockholms läns landsting.